THOUSANDS OF INSULIN PUMPS RECALLED
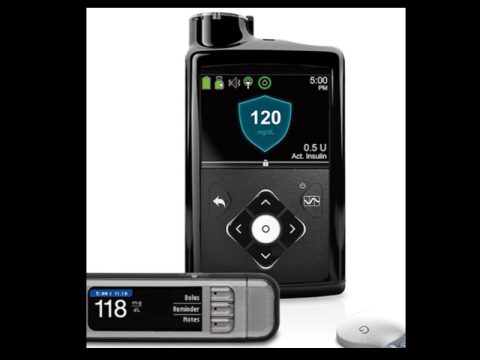
The Food and Drug Administration (FDA) has issued a recall for an insulin pump that thousands of people use with Type 1 diabetes. The recall involves certain Medtronic MiniMed 600 series insulin pumps.
The FDA says one person has died, 2,175 people have received injuries and there have been more than 26,000 complaints.
Medtronic is recalling the specified insulin pumps because of a missing or broken retainer ring. That ring helps lock the insulin cartridge into place. Medtronic said, if the cartridge is not locked into place, the device may under or over-deliver the correct amount of insulin, which could result in hypoglycemia or hyperglycemia. Severe hyperglycemia can result in a loss of consciousness, seizure, and death.
The recall products include:
MiniMed 600 Series Insulin Pumps
Model 630G (MMT-1715) – all lots before October 2019
Model 670G (MMT-1780) – all lots before August 2019
Distribution Dates:
Model 630G – September 2016 to October 2019
Model 670G – June 2017 to August 2019
Medtronic notified affected costumers and health care providers are working with those patients.