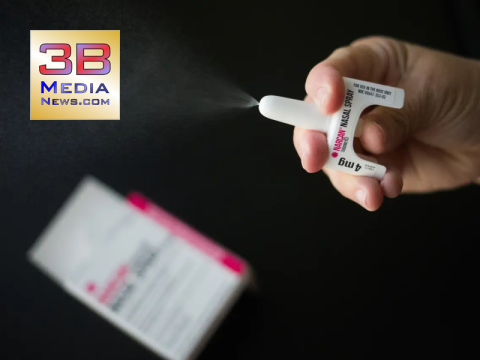
FDA APPROVES OVER-THE-COUNTER NARCAN TO REDUCE OPIOID OVERDOSES
As of March 29th, the FDA approved Narcan, 4 milligrams (mg) of naloxone hydrochloride nasal spray for over-the-counter (OTC), nonprescription, use – the first naloxone product approved for use without a prescription. Naloxone is a medication that rapidly reverses the effects of opioid overdose and is the standard treatment for opioid overdose.
This potential life-saving medication will be sold directly to consumers in places like drug stores, convenience stores, grocery stores, and gas stations, as well as online.
The timeline for the availability and price of this OTC product is determined by the manufacturer.
The Tennessee Department of Mental Health and Substance Abuse Services (TDMHSAS) applauds the Food and Drug Administration’s decision to make the opioid overdose reversal drug Narcan available without a prescription. Tennessee has had a statewide collaborative pharmacy practice agreement in place since 2016 allowing pharmacists to dispense naloxone to customers without a conventional prescription, but this decision at the federal level will expand the availability of the life-saving medication even further.