THYROID MEDICATION RECALLED ACROSS UNITED STATES
The Food and Drug Administration says two types of thyroid medications are being voluntarily recalled by the manufacturer. The NP Thyroid tablets made by Acella Pharmaceuticals, LLC have possibly led to at least four reports of adverse effects. Several problems, including heart issues, could be associated with the recalled drugs. The prescription drugs were sent to pharmacies across the country. Patients who are currently taking NP Thyroid® from the lots being recalled should not discontinue use without contacting their healthcare provider for further guidance and/or a replacement prescription.
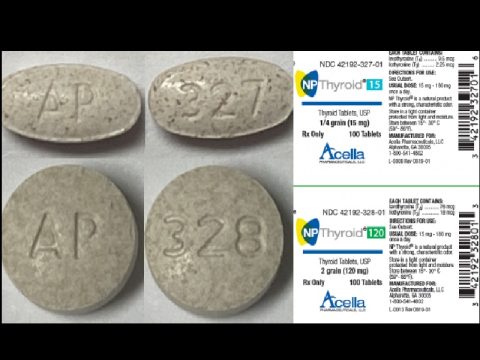
The tablets recalled include:
NP Thyroid® 15, Thyroid Tablets, USP, ¼ grain (15 mg) 42192-327-01 M327E19-1 Expires: October 2020
NP Thyroid® 120, Thyroid Tablets, USP, 2 grain (120 mg) 42192-328-01 M328F19-3 Expires: November 2020